Abstract
Background . Anti-CD19 chimeric antigen receptor T cells (CART19) are now a standard treatment for patients (pts) with relapsed/refractory (r/r) large B-cell lymphomas (LBCL). Lymphodepleting chemotherapy (LD) is administered before CART19 to optimize CAR T cell engraftment, expansion, and function. The most widely used LD regimen is the combination of fludarabine (25-30mg/m 2) and cyclophosphamide (250-500mg/m 2) administered daily over 3 days (Flu/Cy). However, Flu/Cy is associated with a significant risk of hematologic toxicity that may preclude administration or result in prolonged cytopenias in pts with pre-existing cytopenias. Bendamustine (Benda) combines both alkylating-agent and purine-analog activities, and has potent anti-tumor efficacy in lymphoid malignancies. Importantly, compared to Flu/Cy, Benda typically has less hematologic toxicity, which may reduce the risk of infections. Therefore, because of its safety profile and lymphocytotoxic activity, Benda has been used as an alternative LD regimen for some pts receiving tisagenlecleucel (tisa-cel). In this study, we compare outcomes for Benda 90mg/m 2 for 2 days with Flu/Cy as the LD regimen before tisa-cel in pts with r/rLBCL treated at 3 different institutions.
Methods : We retrospectively evaluated the outcomes of 133 consecutive r/r LBCL pts treated with commercial tisa-cel at the Hospital of the University of Pennsylvania, Oregon Health and Science University, and the Medical University of Vienna between 2018 and 2021. Patients with complete response (CR) at the time of infusion (n=20) were excluded from this analysis as this study aimed at evaluating the role of the LD regimen not only as related to the LD ability but also its effect against the tumor. Therefore, the analysis included 113 adult r/rLBCL pts treated with Flu/Cy or Benda as LD and with measurable disease on the last PET/CT scan before tisa-cel infusion. LD choice was based on physician's preference. Pts were evaluated for response (Lugano criteria), progression (PFS) and overall survival (OS), as well as hematological and CART-specific toxicities (ASTCT criteria). Pts demographics, response rates, and adverse events were compared using chi-squared and t-student tests as appropriate; log rank test was used for survival analysis.
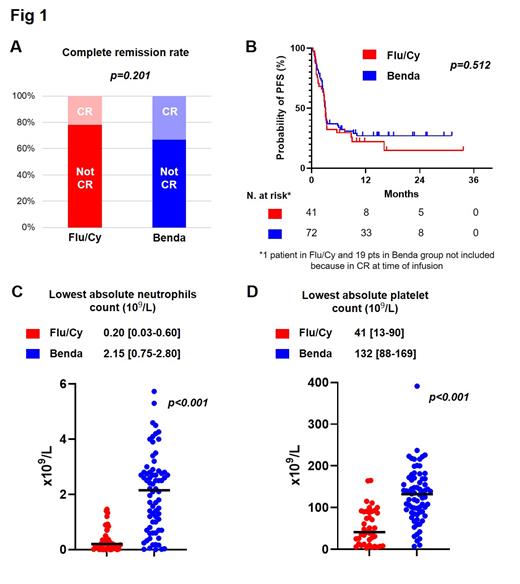
Results: Of 113 pts, 68 (60%) had diffuse large BCL not otherwise specified (NOS), 3 (3%) high-grade BCL NOS, 32 (28%) transformed follicular lymphoma, 9 (8%) high-grade BCL with MYC and BCL2 and/or BCL6 translocations, and 1 (1%) primary mediastinal BCL. Forty-one pts (36%) received Flu/Cy and 72 (64%) received Benda LD. Characteristics of Flu/Cy pts were comparable to Benda in terms of sex (female: 37% vs. 32%, p=0.616), age (68 vs. 65 years, p=0.143), performance status (ECOG ≤1: 93% vs. 94%, p=0.709), number of previous lines of therapy (3 vs. 3, p=0.707), previous autologous hematopoietic cell transplant (27% vs. 14%, p=0.089), bridging therapy (73% vs 85% p=0.136), LDH at infusion (elevated: 54% vs. 51%, p=0.708), and bulky disease (>10 cm) (15% vs. 10%, p=0.431). In whole cohort, no difference in obtaining a CR at any point after CART was observed between groups (Flu/Cy: 22% vs. Benda: 33%, p=0.201) (Fig 1A). At a median follow-up of 20.4 months, no difference in PFS was observed between Flu/Cy and Benda pts with 12-month PFS of 22% and 27%, respectively (p=0.512, Fig 1B). OS was also similar between Flu/Cy and Benda groups (2-year OS 41% vs. 49%, respectively, p=0.108).
Both cytokine-release syndrome (CRS) and neurotoxicity (ICANS) were more frequent in the Flu/Cy group compared to Benda (any grade CRS: 68% vs. 40%, respectively, p=0.004; any grade ICANS: 22% vs. 7%, respectively, p=0.020). Of note, pts receiving Flu/Cy developed more severe cytopenias compared to Benda. In particular, the median absolute neutrophil count nadir 30 days after tisa-cel was significantly lower in Flu/Cy group (0.20x10 9/L) compared to Benda (2.15x10 9/L) (p<0.001). Similarly, median platelet count nadir was lower in Flu/Cy pts compared to Benda (41x10 9/L vs. 132x10 9/L, p<0.001) (Fig 1C-D). A subset analysis in DLBCL-NOS patients confirmed no difference in efficacy and increased hematological toxicity in the Flu/Cy group.
Conclusions: This retrospective study of r/r LBCL pts receiving tisagenlecleucel suggests that Benda is as effective as Flu/Cy and validates a safer adverse event profile with reduced CRS, ICANS, and hematological toxicities.
Svoboda: Pharmacyclics: Consultancy, Research Funding; TG: Research Funding; Seattle Genetics: Consultancy, Research Funding; Imbrium: Consultancy; Merck: Research Funding; Incyte: Research Funding; Genmab: Consultancy; Atara: Consultancy; BMS: Consultancy, Research Funding; Astra Zeneca: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding. Dwivedy Nasta: Merck: Other: Data safety monitoring board; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; ATARA: Research Funding; Millenium: Research Funding; Pharmacyclics: Research Funding; Roche: Research Funding; Rafael: Research Funding; Debiopharm: Research Funding. Landsburg: Incyte: Membership on an entity's Board of Directors or advisory committees; ADCT: Membership on an entity's Board of Directors or advisory committees; Curis: Research Funding; Takeda: Research Funding; Triphase: Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Other: DSMB member; Morphosys: Membership on an entity's Board of Directors or advisory committees. Gerson: Kite: Consultancy; TG Therapeutics: Consultancy; Abbvie: Consultancy; Pharmacyclics: Consultancy. Barta: Daiichi Sankyo: Honoraria; Seagen: Honoraria; Acrotech: Honoraria; Kyowa Kirin: Honoraria. Garfall: Amgen: Honoraria; Tmunity Therapeutics: Research Funding; Janssen: Honoraria, Research Funding; GSK: Honoraria; Novartis: Research Funding. Porter: National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Genentech: Current equity holder in publicly-traded company, Ended employment in the past 24 months; American Society for Transplantation and Cellular Therapy: Honoraria; Incyte: Membership on an entity's Board of Directors or advisory committees; DeCart: Membership on an entity's Board of Directors or advisory committees; ASH: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Tmunity: Patents & Royalties; Wiley and Sons Publishing: Honoraria. Jaeger: BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Norvartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees. Maziarz: Novartis: Consultancy, Other: Data and Safety Monitoring board, Research Funding; Bristol-Myers, Squibb/Celgene,, Intellia, Kite: Honoraria; Incyte Corporation: Consultancy, Honoraria; Allovir: Consultancy, Research Funding; Artiva Therapeutics: Consultancy; CRISPR Therapeutics: Consultancy; Intellia: Honoraria; Omeros: Research Funding; Athersys: Other: Data and Safety Monitoring Board, Patents & Royalties; Vor Pharma: Other: Data and Safety Monitoring Board. Ruella: AbClon: Consultancy, Research Funding; viTToria biotherapeutics: Research Funding; Tmunity: Patents & Royalties; BMS, BAYER, GSK: Consultancy; Novartis: Patents & Royalties. Schuster: Abbvie: Consultancy, Research Funding; Acerta Pharma: Consultancy; AstraZeneca: Consultancy; Adaptive Biotechnologies: Research Funding; BeiGene: Consultancy; Celgene: Consultancy, Honoraria, Research Funding; DTRM: Research Funding; Genetech: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Incyte: Research Funding; Juno Theraputics: Consultancy, Research Funding; Loxo Oncology: Consultancy; Merck: Research Funding; Nordic Nanovector: Consultancy; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding; Pharmaclcyclics: Research Funding; Tessa Theraputics: Consultancy; TG Theraputics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal